A solution purpose-built for the enterprise
With MessageGears, you can do pretty much anything you can think about, and the technology just gets out of the way. It’s genuinely changed what we can do as a company.
Mark Stange-Tregear
VP Analytics at Rakuten

THE MESSAGEGEARS PLATFORM
Execute the cross-channel customer experience you’ve always dreamed of delivering – without all the roadblocks
Scale your 1:1 personalization with instant access to unlimited data points – without the extra hassle or fees.
Leverage any data point to create sophisticated audiences - and activate them across any channel, fast.
Create deeper customer connections by delivering unified, in-the-moment experiences across mobile, email, SMS, and web.

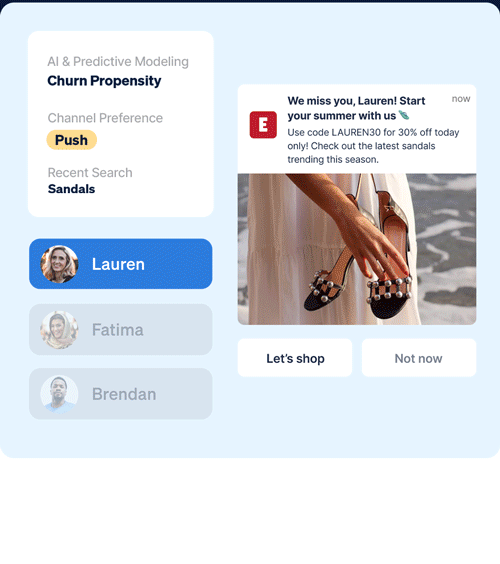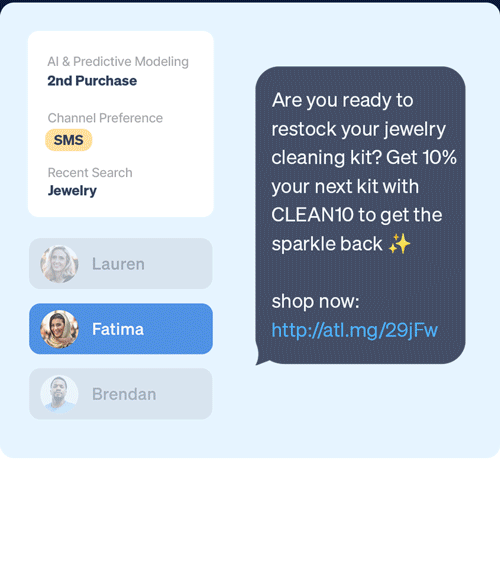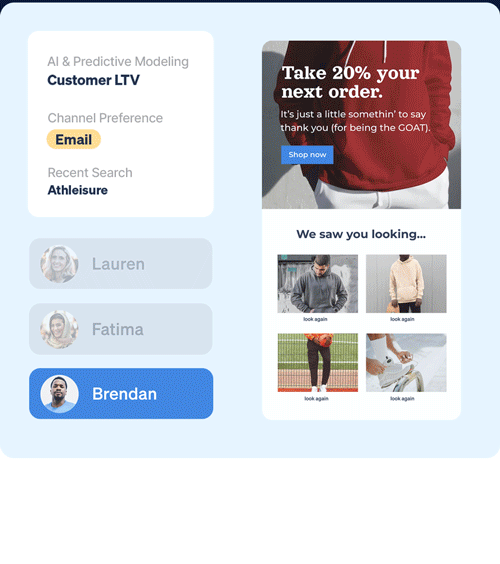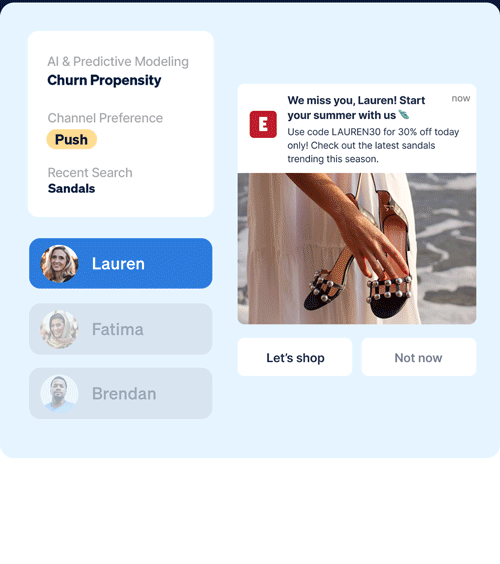
Identify which customers are most likely to take (or not take) critical lifecycle actions with predictive AI that’s designed to optimize every touch point.
MessageGears integrates with the tech you already know and love
Why enterprise brands trust MessageGears
News & Resources